Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal.Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas.Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals
1428 questions with answers in POLYMER CHEMISTRY | Science topic
Crystallization is a technique used for the purification of substances. A separation technique to separate solids from a solution. Crystallization can be defined as the process through which the atoms/molecules of a substance arrange themselves in a well-defined three-dimensional lattice and consequently, minimize the overall energy of the system.

Source Image: mdpi.com
Download Image
A reactive crystallization could be performed for instance by adding a high pH solution to a low pH solution of a compound: the change in pH upon mixing the solutions reduces the solubility of the compound and crystallization can occur. A continuous reactive crystallization would have two solution feeds and a suspension outflow.
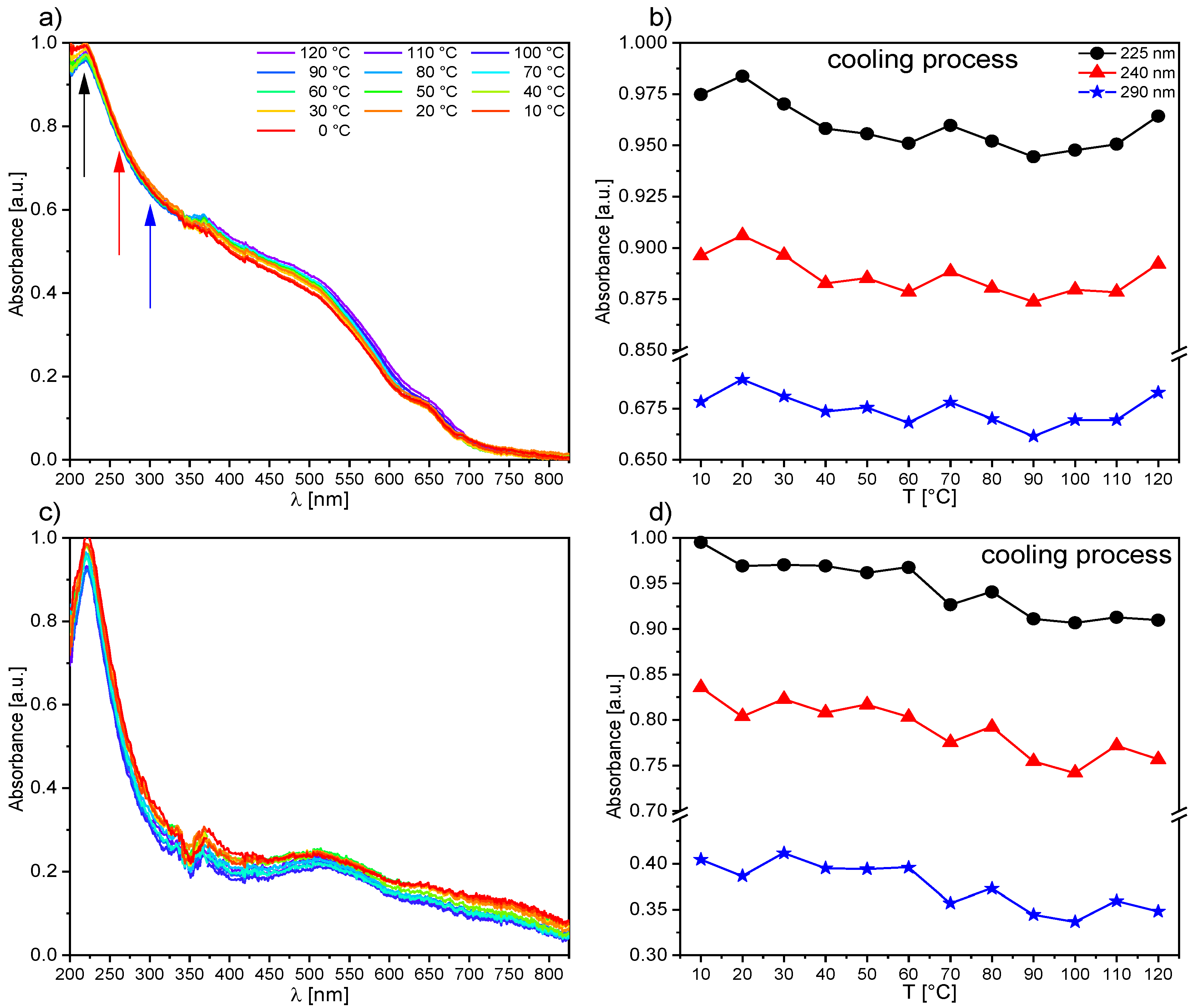
Source Image: mdpi.com
Download Image
Atrasentan Revisited | New Drug Approvals Add a “seed crystal”: a small speck of crude solid saved from before the crystallization was begun, or a bit of pure solid from a reagent jar. Seed crystals create a nucleation site where crystals can begin growth. Dip a glass stirring rod into the supersaturated solution, remove it, and allow the solvent to evaporate to produce a thin residue

Source Image: numerade.com
Download Image
Crystallization Of A Pure Compound Is Spontaneous Only Below
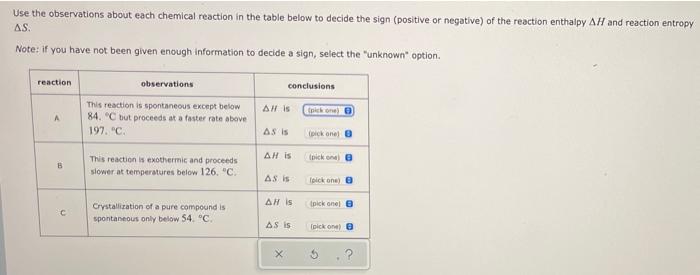
Add a “seed crystal”: a small speck of crude solid saved from before the crystallization was begun, or a bit of pure solid from a reagent jar. Seed crystals create a nucleation site where crystals can begin growth. Dip a glass stirring rod into the supersaturated solution, remove it, and allow the solvent to evaporate to produce a thin residue Crystallization of a pure compound is spontaneous only below 109. °C. This reaction is exothermic and proceeds faster at temperatures above 34. °C. conclusions AH is AS is AH is AS is AH is AS is (pick one) (pick one) (pick one) (pick one) (pick one) (pick one)
SOLVED: Use the observations bout each chemica reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy 4S, Note: if you have
Jan 30, 2023Recrystallization, also known as fractional crystallization, is a procedure for purifying an impure compound in a solvent. The method of purification is based on the principle that the solubility of most solids increases with increased temperature. This means that as temperature increases, the amount of solute that can be dissolved in a solvent Molecules | Free Full-Text | Statistical Analysis of Copper(I) Iodide and Bis(Diphenylphosphino)alkane-Based Complexes and Coordination Polymers

Source Image: mdpi.com
Download Image
Acta Phys. -Chim. Sin. Jan 30, 2023Recrystallization, also known as fractional crystallization, is a procedure for purifying an impure compound in a solvent. The method of purification is based on the principle that the solubility of most solids increases with increased temperature. This means that as temperature increases, the amount of solute that can be dissolved in a solvent
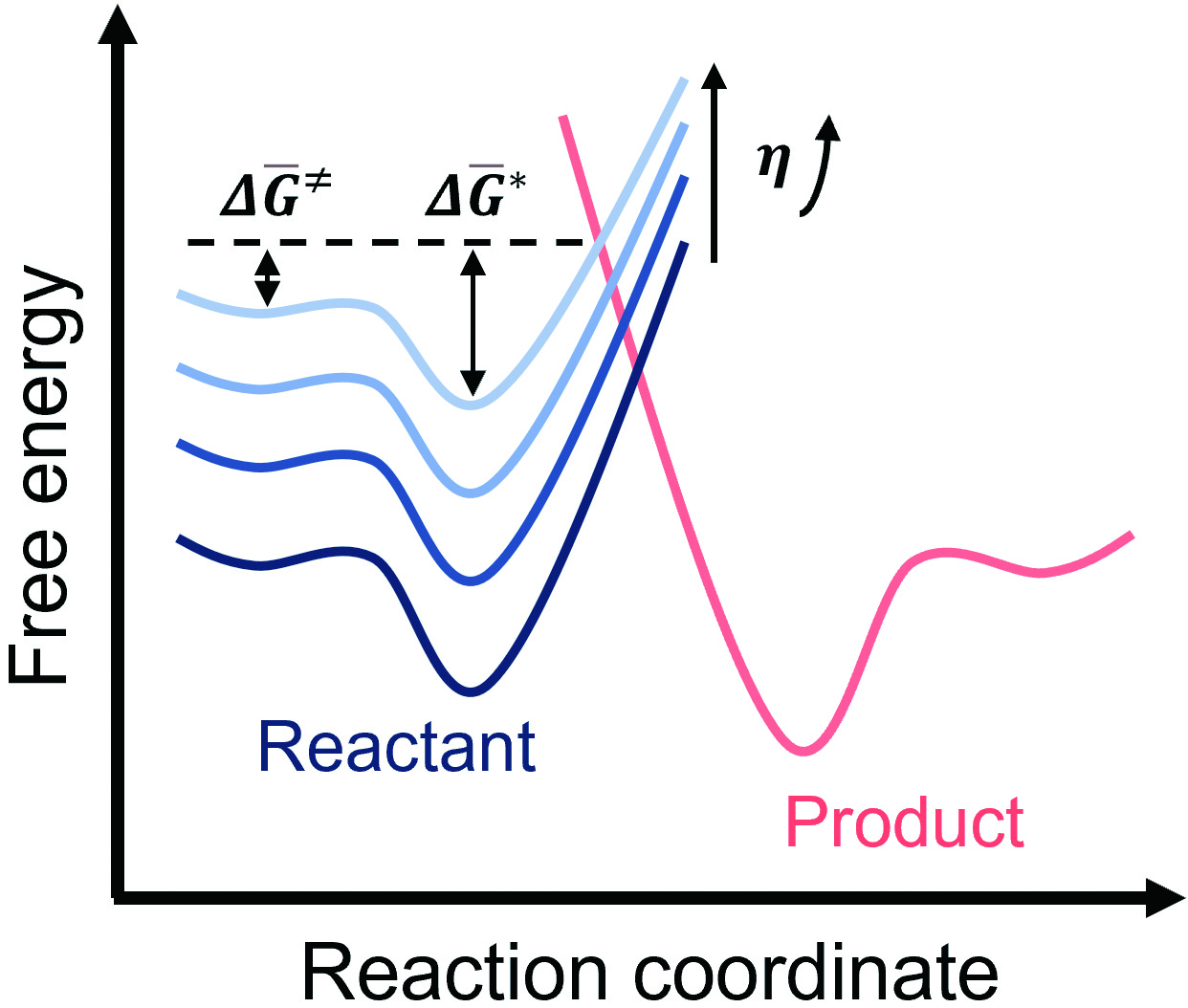
Source Image: whxb.pku.edu.cn
Download Image
1428 questions with answers in POLYMER CHEMISTRY | Science topic Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal.Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas.Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals

Source Image: researchgate.net
Download Image
Atrasentan Revisited | New Drug Approvals A reactive crystallization could be performed for instance by adding a high pH solution to a low pH solution of a compound: the change in pH upon mixing the solutions reduces the solubility of the compound and crystallization can occur. A continuous reactive crystallization would have two solution feeds and a suspension outflow.

Source Image: newdrugapprovals.org
Download Image
Solved Use the observations about each chemical reaction in | Chegg.com Crystallization is a technique which chemists use to purify solid compounds. It is one of the fundamental procedures each chemist must master to become proficient in the laboratory. Crystallization is based on the principles of solubility: compounds (solutes) tend to be more soluble in hot liquids (solvents) than they are in cold liquids.
Source Image: chegg.com
Download Image
Answered: reaction A B C observations This… | bartleby Add a “seed crystal”: a small speck of crude solid saved from before the crystallization was begun, or a bit of pure solid from a reagent jar. Seed crystals create a nucleation site where crystals can begin growth. Dip a glass stirring rod into the supersaturated solution, remove it, and allow the solvent to evaporate to produce a thin residue

Source Image: bartleby.com
Download Image
Minimizing Polymorphic Risk through Cooperative Computational and Experimental Exploration | Journal of the American Chemical Society Crystallization of a pure compound is spontaneous only below 109. °C. This reaction is exothermic and proceeds faster at temperatures above 34. °C. conclusions AH is AS is AH is AS is AH is AS is (pick one) (pick one) (pick one) (pick one) (pick one) (pick one)

Source Image: pubs.acs.org
Download Image
Acta Phys. -Chim. Sin.
Minimizing Polymorphic Risk through Cooperative Computational and Experimental Exploration | Journal of the American Chemical Society Crystallization is a technique used for the purification of substances. A separation technique to separate solids from a solution. Crystallization can be defined as the process through which the atoms/molecules of a substance arrange themselves in a well-defined three-dimensional lattice and consequently, minimize the overall energy of the system.
Atrasentan Revisited | New Drug Approvals Answered: reaction A B C observations This… | bartleby Crystallization is a technique which chemists use to purify solid compounds. It is one of the fundamental procedures each chemist must master to become proficient in the laboratory. Crystallization is based on the principles of solubility: compounds (solutes) tend to be more soluble in hot liquids (solvents) than they are in cold liquids.