Jun 23, 2023Watch on Steps of drawing ClBr3 lewis structure Step 1: Find the total valence electrons in ClBr3 molecule In order to find the total valence electrons in a ClBr3 molecule, first of all you should know the valence electrons present in the chlorine atom as well as bromine atom.
Draw the Lewis structure of ClBr3 with lone pairs. | Homework.Study.com
Draw the lewis structure of clbr3 showing all lone pairs. | Quizlet Related questions with answers Draw the Lewis dot structure of \ce SiBr4 SiBrX 4. Classify these diatomic molecules as diamagnetic or paramagnetic: O2, F2, B2, C2, N2 Which of the atoms in the molecular models in the earlier Problem have: a. three lone pairs b. two lone pairs
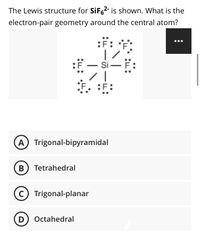
Source Image: bartleby.com
Download Image
Compute answers using Wolfram’s breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history
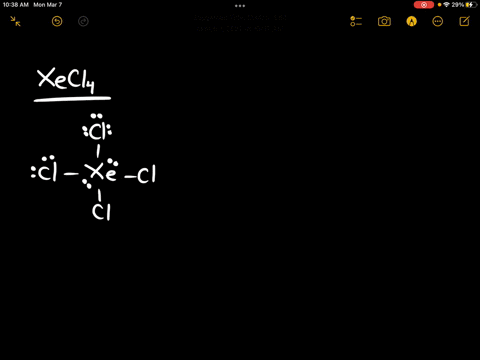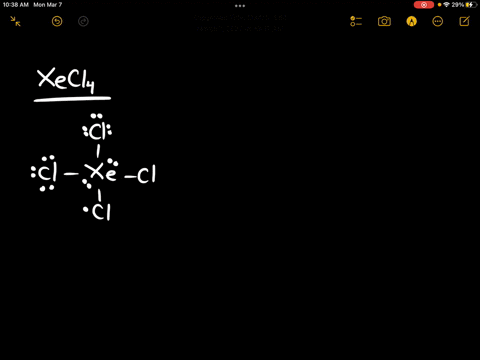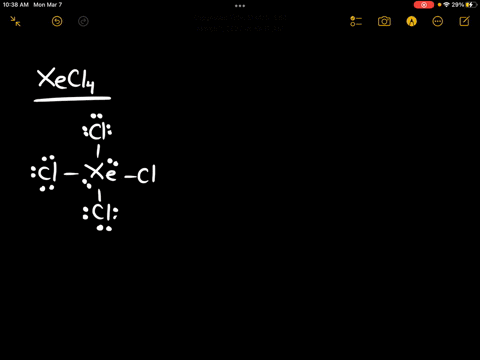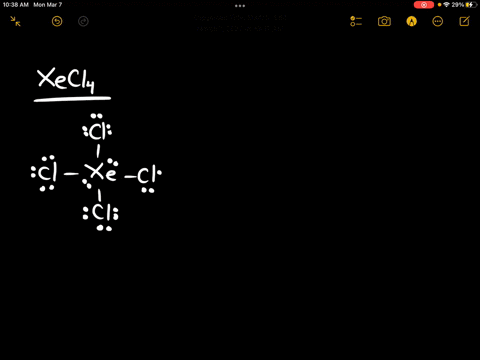
Source Image: numerade.com
Download Image
For the tellurium diiodide molecule determine the molecular formula, Lewis structure, shape, polarity, and intermolecular force. | Homework.Study.com In Lewis structures the bonding pair of electrons is usually displayed as a line, and the unshared electrons as dots: … Draw the Lewis Structure for the chlorate ion (ClO 3-). … Charges of -1 and +1 on adjacent atoms can usually be removed by using a lone pair of electrons from the -1 atom to form a double (or triple) bond to the atom with

Source Image: brainly.com
Download Image
Draw The Lewis Structure Of Clbr3 Showing All Lone Pairs
In Lewis structures the bonding pair of electrons is usually displayed as a line, and the unshared electrons as dots: … Draw the Lewis Structure for the chlorate ion (ClO 3-). … Charges of -1 and +1 on adjacent atoms can usually be removed by using a lone pair of electrons from the -1 atom to form a double (or triple) bond to the atom with 1. Determine the total number of valence electrons in ClBr3. – Chlorine (Cl) has 7 valence electrons. – Bromine (Br) has 7 valence electrons. – There are 3 bromine atoms, so the total number of valence electrons for bromine is 7 x 3 = 21. – The total number of valence electrons for ClBr3 is 7 + 21 = 28. Step 2/5 2. Determine the central atom.
draw the lewis structure of the following molecule include lone pairs NCl3 – brainly.com
Nov 13, 2023Steps To properly draw the ClBr 3 Lewis structure, follow these steps: #1 Draw a rough sketch of the structure #2 Next, indicate lone pairs on the atoms #3 Indicate formal charges on the atoms, if necessary Let’s break down each step in more detail. #1 Draw a rough sketch of the structure First, determine the total number of valence electrons Answered: What is the molecular geometry of these… | bartleby
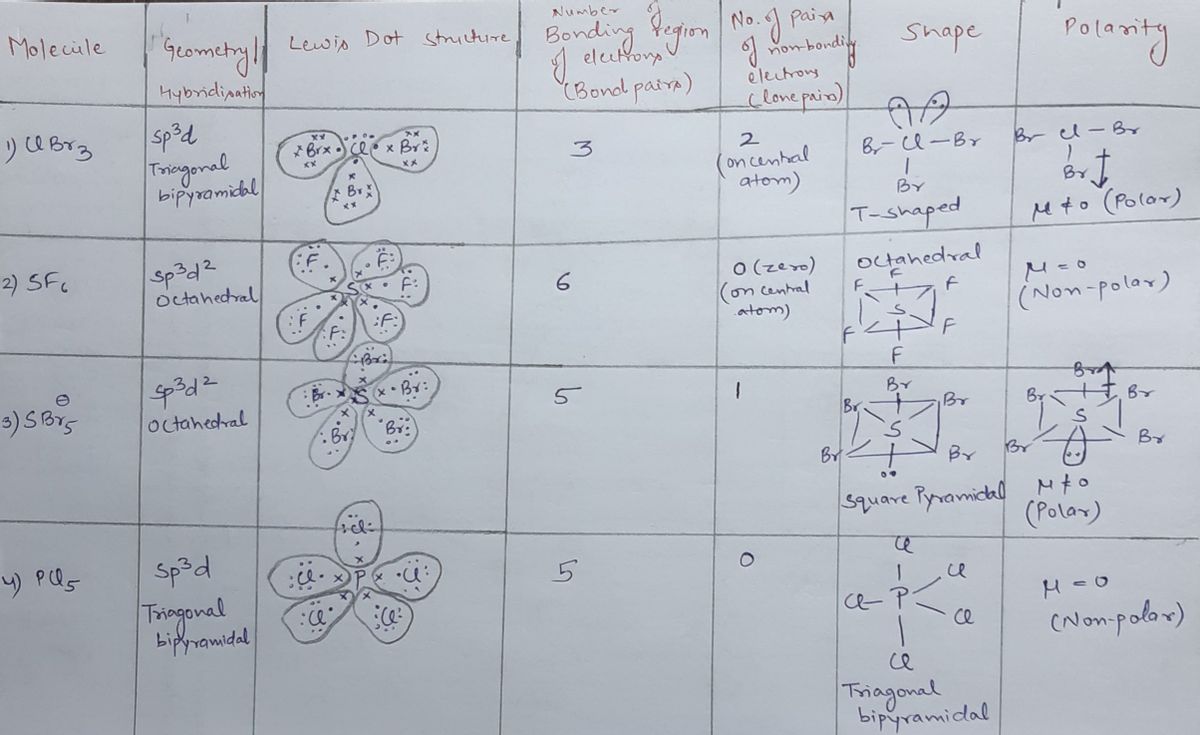
Source Image: bartleby.com
Download Image
Draw the Lewis structure for OPBr_3. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons. | Homework.Study.com Nov 13, 2023Steps To properly draw the ClBr 3 Lewis structure, follow these steps: #1 Draw a rough sketch of the structure #2 Next, indicate lone pairs on the atoms #3 Indicate formal charges on the atoms, if necessary Let’s break down each step in more detail. #1 Draw a rough sketch of the structure First, determine the total number of valence electrons

Source Image: homework.study.com
Download Image
Draw the Lewis structure of ClBr3 with lone pairs. | Homework.Study.com Jun 23, 2023Watch on Steps of drawing ClBr3 lewis structure Step 1: Find the total valence electrons in ClBr3 molecule In order to find the total valence electrons in a ClBr3 molecule, first of all you should know the valence electrons present in the chlorine atom as well as bromine atom.

Source Image: homework.study.com
Download Image
For the tellurium diiodide molecule determine the molecular formula, Lewis structure, shape, polarity, and intermolecular force. | Homework.Study.com Compute answers using Wolfram’s breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history

Source Image: homework.study.com
Download Image
Describe how to find total pairs, shared pairs, and lone pairs after creating the Lewis Structures? | Homework.Study.com Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.

Source Image: homework.study.com
Download Image
The structure of TeF5^- is below-mentioned. Draw a complete Lewis structure for TeF5^-, and explain the distortion from the ideal square pyramidal structure. | Homework.Study.com In Lewis structures the bonding pair of electrons is usually displayed as a line, and the unshared electrons as dots: … Draw the Lewis Structure for the chlorate ion (ClO 3-). … Charges of -1 and +1 on adjacent atoms can usually be removed by using a lone pair of electrons from the -1 atom to form a double (or triple) bond to the atom with

Source Image: homework.study.com
Download Image
Solved] Draw the Lewis structure of ClBr3 with lone pairs. | Course Hero 1. Determine the total number of valence electrons in ClBr3. – Chlorine (Cl) has 7 valence electrons. – Bromine (Br) has 7 valence electrons. – There are 3 bromine atoms, so the total number of valence electrons for bromine is 7 x 3 = 21. – The total number of valence electrons for ClBr3 is 7 + 21 = 28. Step 2/5 2. Determine the central atom.
Source Image: coursehero.com
Download Image
Draw the Lewis structure for OPBr_3. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons. | Homework.Study.com
Solved] Draw the Lewis structure of ClBr3 with lone pairs. | Course Hero Draw the lewis structure of clbr3 showing all lone pairs. | Quizlet Related questions with answers Draw the Lewis dot structure of \ce SiBr4 SiBrX 4. Classify these diatomic molecules as diamagnetic or paramagnetic: O2, F2, B2, C2, N2 Which of the atoms in the molecular models in the earlier Problem have: a. three lone pairs b. two lone pairs
For the tellurium diiodide molecule determine the molecular formula, Lewis structure, shape, polarity, and intermolecular force. | Homework.Study.com The structure of TeF5^- is below-mentioned. Draw a complete Lewis structure for TeF5^-, and explain the distortion from the ideal square pyramidal structure. | Homework.Study.com Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.