The Diels–Alder reaction is most useful for synthesizing molecules in the lab. But scientists believe that specific enzymes catalyze Diels–Alder reactions in some organisms. Examples are the formation of lovastatin, a cholesterol-lowering drug found in oyster mushrooms, and Spinosyn A, a natural insecticide produced by a certain bacterium. Comment.
In each Diels–Alder reaction shown, predict the product that will… | Channels for Pearson+
Therefore, it is the thermodynamic product (more stable) and the endo is the kinetic product (forms faster). *Note: The orbitals are shown in a simple way without indicating the phases. This is more complicated than it looks and there are challenges in favor and against any arguments. Relationship of products in the Diels–Alder reaction
Source Image: masterorganicchemistry.com
Download Image
Asymmetric Diels–Alder. Substrates with bulky substituents will affect the diastereoselectivity of a Diels–Alder reaction by limiting the approach of the diene/dienophile pair. Here, we see the preferred endo product that minimizes steric interactions with the phenyl substituent (Synthesis 2002, 2457-2463).

Source Image: pearson.com
Download Image
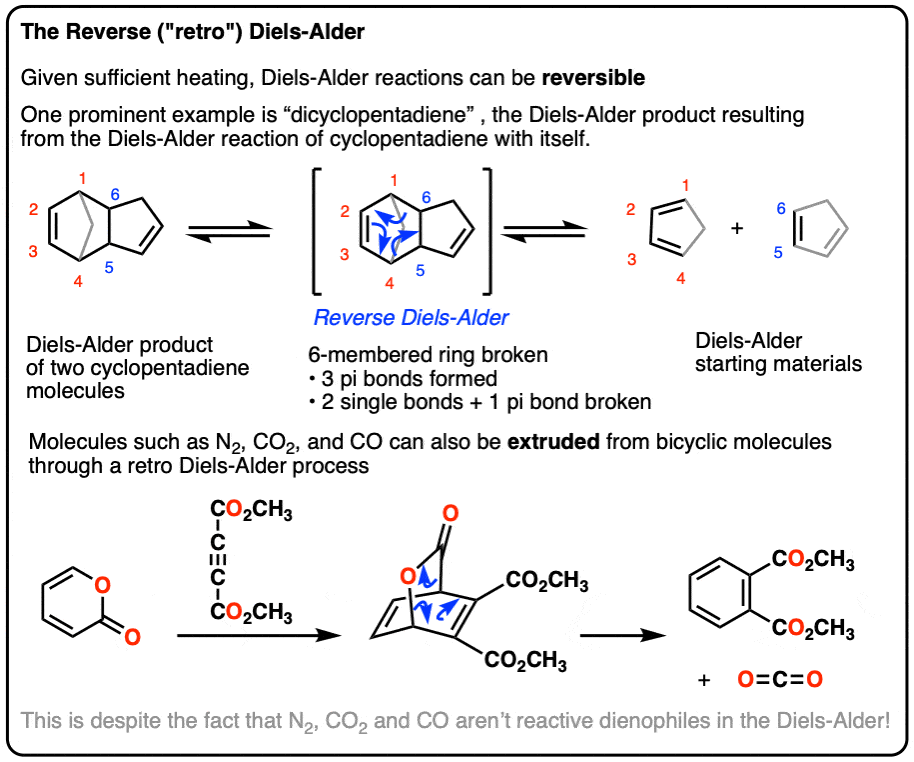
Solved 2. Identify the expected major product of the | Chegg.com The simplest Diels–Alder reaction uses 1,3-butadiene and ethene. Ethene (the dienophile) performs a Diels–Alder reaction with 1,3-butadiene (the diene) to form cyclohexene. The diene, so-called by chemists due to its two double bonds, supplies four of the six carbons to the cyclic product. Interestingly, the diene’s two double bonds must be
Source Image: coursehero.com
Download Image
Identify The Expected Major Product Of The Following Diels-Alder Reaction
The simplest Diels–Alder reaction uses 1,3-butadiene and ethene. Ethene (the dienophile) performs a Diels–Alder reaction with 1,3-butadiene (the diene) to form cyclohexene. The diene, so-called by chemists due to its two double bonds, supplies four of the six carbons to the cyclic product. Interestingly, the diene’s two double bonds must be Furan and maleimide, shown below, react to produce and adduct via a Diels–Alder reaction. At 25°C the isomer produced is the endo product, however at 90°C the exo isomer predominates. Additional studies have shown that at 90°C the equilibrium between the endo and exo products favors the exo isomer. O. a) Draw each isomeric product, endo and exo.
Solved] . 9. Predict the major product for the following Diels-Alder… | Course Hero
Figure 10.3.3 10.3. 3: A Diels Alder reaction and its reverse. Typically, Diels Alder reactions occur at low or moderate temperatures (between 25 °C and 100 °C is a common range). In comparison, retro Diels Alder reactions require more elevated temperatures, often above 200 °C. The forward reaction is favored at low temperature, whereas the Predict the products of the following proposed Diels–Alder reacti… | Channels for Pearson+

Source Image: pearson.com
Download Image
Solved Identify the expected major product of the following | Chegg.com Figure 10.3.3 10.3. 3: A Diels Alder reaction and its reverse. Typically, Diels Alder reactions occur at low or moderate temperatures (between 25 °C and 100 °C is a common range). In comparison, retro Diels Alder reactions require more elevated temperatures, often above 200 °C. The forward reaction is favored at low temperature, whereas the
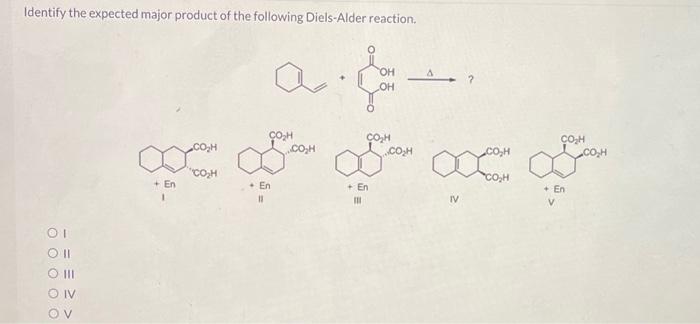
Source Image: chegg.com
Download Image
In each Diels–Alder reaction shown, predict the product that will… | Channels for Pearson+ The Diels–Alder reaction is most useful for synthesizing molecules in the lab. But scientists believe that specific enzymes catalyze Diels–Alder reactions in some organisms. Examples are the formation of lovastatin, a cholesterol-lowering drug found in oyster mushrooms, and Spinosyn A, a natural insecticide produced by a certain bacterium. Comment.

Source Image: pearson.com
Download Image
Solved 2. Identify the expected major product of the | Chegg.com Asymmetric Diels–Alder. Substrates with bulky substituents will affect the diastereoselectivity of a Diels–Alder reaction by limiting the approach of the diene/dienophile pair. Here, we see the preferred endo product that minimizes steric interactions with the phenyl substituent (Synthesis 2002, 2457-2463).
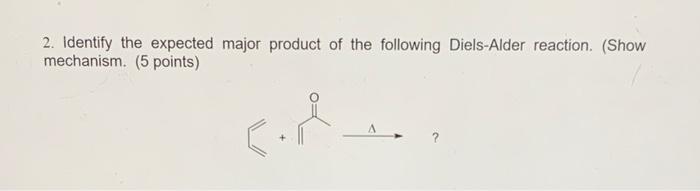
Source Image: chegg.com
Download Image
Enzymatic intermolecular Diels-Alder reactions in synthesis: From nature to design – ScienceDirect DielsAlder reaction Diels–Alder: stereochemistry of dienophile Diels–Alder: stereochemistry of diene Diels–Alder: endo rule Diels–Alder: intramolecular Diels–Alder: regiochemistry Science > Organic chemistry > Conjugated systems and pericyclic reactions > Diels–Alder reaction © 2024 Khan Academy Terms of use Privacy Policy Cookie Notice

Source Image: sciencedirect.com
Download Image
Solved Identify the diene and dienophile expected to produce | Chegg.com The simplest Diels–Alder reaction uses 1,3-butadiene and ethene. Ethene (the dienophile) performs a Diels–Alder reaction with 1,3-butadiene (the diene) to form cyclohexene. The diene, so-called by chemists due to its two double bonds, supplies four of the six carbons to the cyclic product. Interestingly, the diene’s two double bonds must be
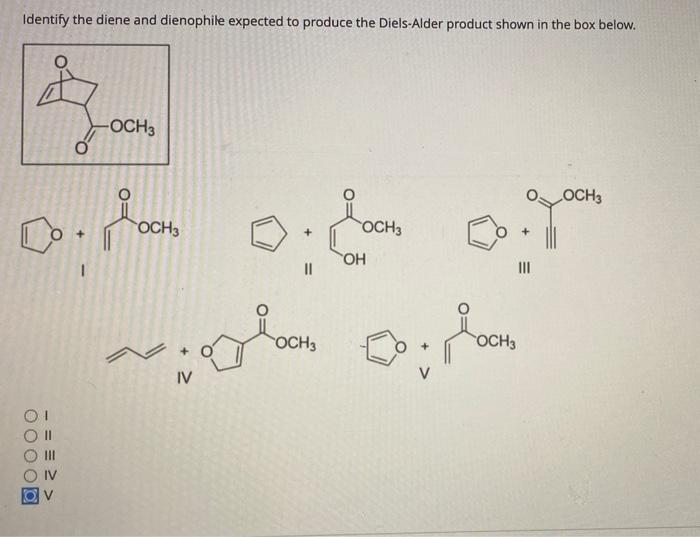
Source Image: chegg.com
Download Image
Diels–Alder reaction – Wikipedia Furan and maleimide, shown below, react to produce and adduct via a Diels–Alder reaction. At 25°C the isomer produced is the endo product, however at 90°C the exo isomer predominates. Additional studies have shown that at 90°C the equilibrium between the endo and exo products favors the exo isomer. O. a) Draw each isomeric product, endo and exo.

Source Image: en.wikipedia.org
Download Image
Solved Identify the expected major product of the following | Chegg.com
Diels–Alder reaction – Wikipedia Therefore, it is the thermodynamic product (more stable) and the endo is the kinetic product (forms faster). *Note: The orbitals are shown in a simple way without indicating the phases. This is more complicated than it looks and there are challenges in favor and against any arguments. Relationship of products in the Diels–Alder reaction
Solved 2. Identify the expected major product of the | Chegg.com Solved Identify the diene and dienophile expected to produce | Chegg.com DielsAlder reaction Diels–Alder: stereochemistry of dienophile Diels–Alder: stereochemistry of diene Diels–Alder: endo rule Diels–Alder: intramolecular Diels–Alder: regiochemistry Science > Organic chemistry > Conjugated systems and pericyclic reactions > Diels–Alder reaction © 2024 Khan Academy Terms of use Privacy Policy Cookie Notice