The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table. The average atomic mass of the element is ________________ amu. Isotope Abundance Mass ———————————— 221x 74.22 220.9 220x 12.78 220.0 218x 13.00 218.1 Click the card to flip 👆 220.4
SOLVED: An undiscovered element has three naturally occurring isotopes of X-55, X-57, and X-58. Isotope X-55 has an abundance of 27.80 % and isotope X-57 has an abundance of 44.39 %.
1. rusting of a nail. 2. freezing of water. 3. decomposition of water into hydrogen and oxygen gases 4. compression of oxygen gas. 1,3. The element X has two naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu.

Source Image: toppr.com
Download Image
Element X has 3 naturally occurring isotopes. The first isotope has an atomic mass of 28.0 amu and a natural abundance of 14.0%. The second isotope has an atomic mass of 29.0 amu and a natural abundance of 66.0%. The third isotope has an atomic mass of 27.4 amu and a natural abundance of 20.0%. What is the atomic mass of element X? a-27.1 amu
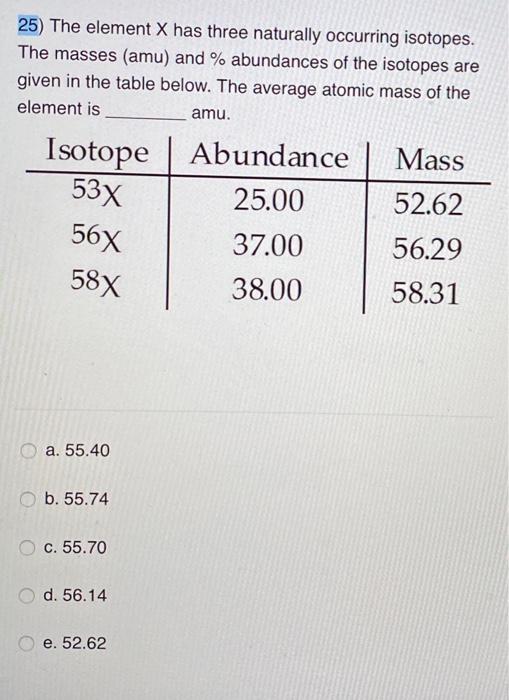
Source Image: chegg.com
Download Image
SOLVED: Element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below: What is the average atomic mass of the element?
The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is amu. Isotope Abundance 221x 82.900 220x 13.200 218x 3.9000 Mass 220.90 220.00 218.10 This problem has been solved!
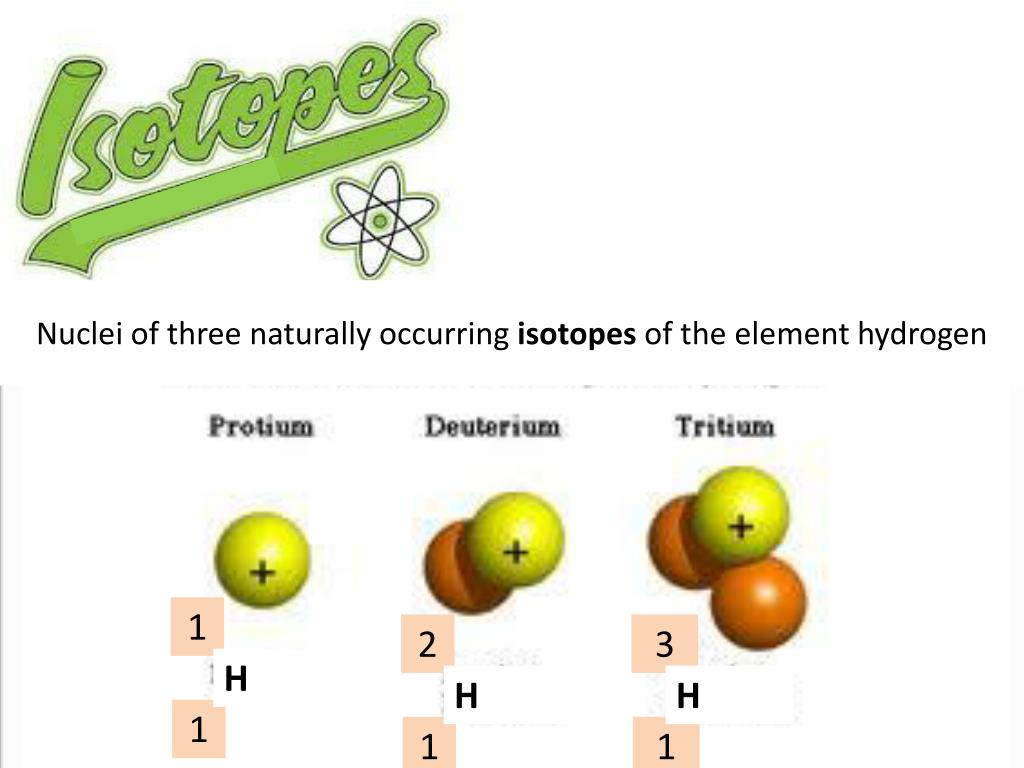
Source Image: slideserve.com
Download Image
Element X Has Three Naturally Occurring Isotopes
The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is amu. Isotope Abundance 221x 82.900 220x 13.200 218x 3.9000 Mass 220.90 220.00 218.10 This problem has been solved!
Every isotope (at least, the ones that occur naturally) contributes to the average atomic mass, which appears in the element’s box on most periodic tables. But the average is what is called a weighted average. A weighted average mass is an average that takes into account how many times each mass occurs in a sample.
PPT – 2.5 Atomic & Nuclear Physics PowerPoint Presentation, free download – ID:4503551
The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ____________amu. Isotope Abundance 221x 7422 12.78 13.00 220x 218x Mass 220.9 220.0 218.1 The element X has three naturally occurring isotopes.
Solved The element X has three naturally occurring isotopes. | Chegg.com

Source Image: chegg.com
Download Image
SOLVED: Element X has two naturally occurring isotopes, 65X (isotopic mass 64.9797 amu, abundance 23.62%) and 67X (isotopic mass 67.1540 amu, abundance 76.38%). Calculate the atomic mass of element X. ???? amu *please show work clearly 🙂
The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ____________amu. Isotope Abundance 221x 7422 12.78 13.00 220x 218x Mass 220.9 220.0 218.1 The element X has three naturally occurring isotopes.
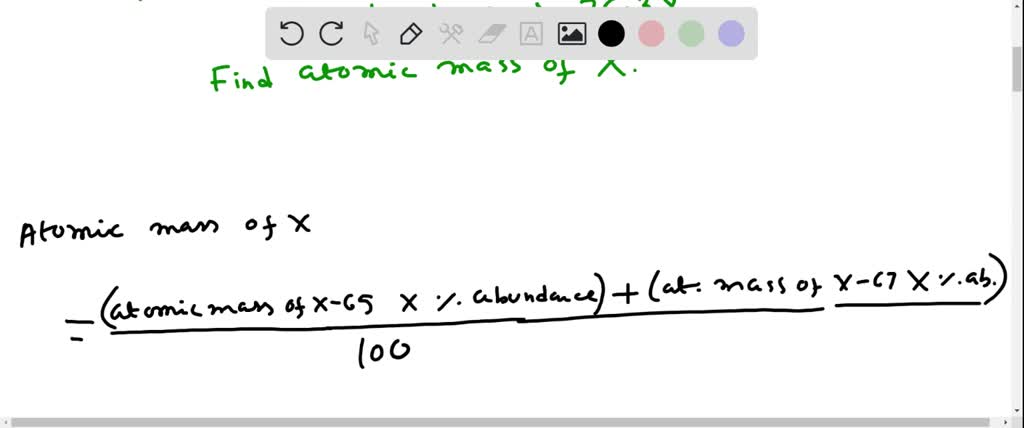
Source Image: numerade.com
Download Image
SOLVED: An undiscovered element has three naturally occurring isotopes of X-55, X-57, and X-58. Isotope X-55 has an abundance of 27.80 % and isotope X-57 has an abundance of 44.39 %.
The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table. The average atomic mass of the element is ________________ amu. Isotope Abundance Mass ———————————— 221x 74.22 220.9 220x 12.78 220.0 218x 13.00 218.1 Click the card to flip 👆 220.4

Source Image: numerade.com
Download Image
SOLVED: Element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below: What is the average atomic mass of the element?
Element X has 3 naturally occurring isotopes. The first isotope has an atomic mass of 28.0 amu and a natural abundance of 14.0%. The second isotope has an atomic mass of 29.0 amu and a natural abundance of 66.0%. The third isotope has an atomic mass of 27.4 amu and a natural abundance of 20.0%. What is the atomic mass of element X? a-27.1 amu
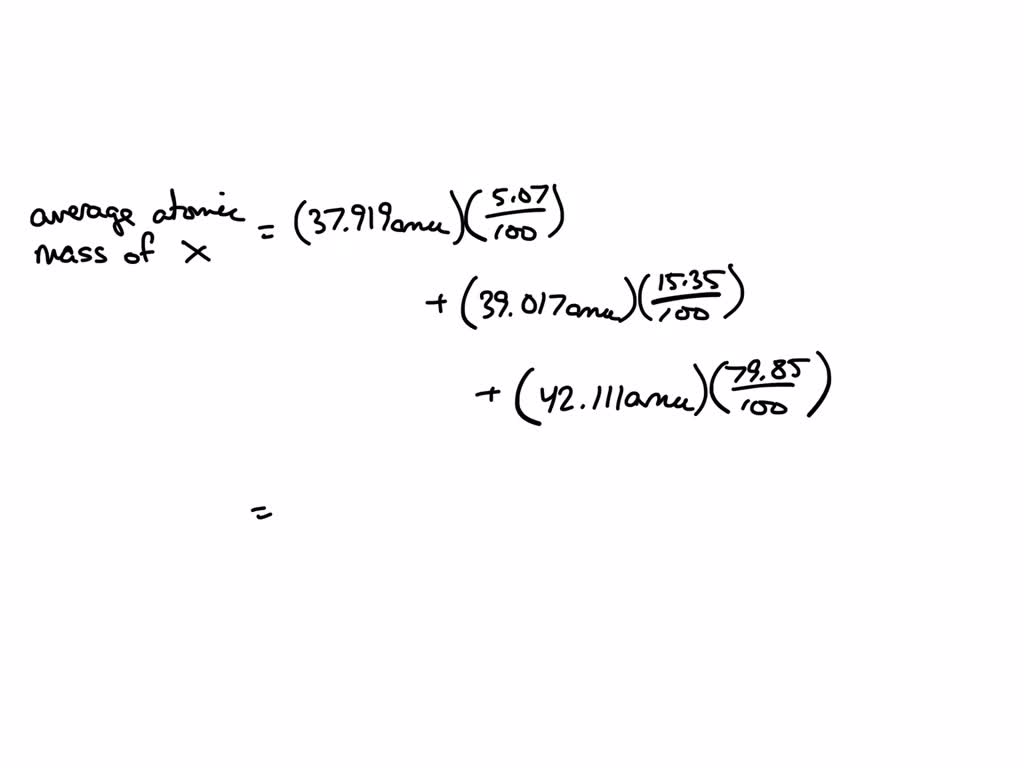
Source Image: numerade.com
Download Image
Solved QUESTION 19 Which pair of atoms constitutes a pair of | Chegg.com
Chemistry Periodic Table of Elements Chemistry-Chapter 2 Practice Problems An unknown element is found to have three naturally occurring isotopes with atomic masses of 35.9675 (0.337%), 37.9627 (0.063%), and 39.9624 (99.600%). Which of the following is the unknown element? Click the card to flip 👆 Ar Click the card to flip 👆 1 / 9 Flashcards Learn

Source Image: chegg.com
Download Image
The Three Isotopes of Hydrogen | Differences & Properties – Video & Lesson Transcript | Study.com
The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is amu. Isotope Abundance 221x 82.900 220x 13.200 218x 3.9000 Mass 220.90 220.00 218.10 This problem has been solved!

Source Image: study.com
Download Image
Silicon has three naturally occurring isotopes (Si-28, Si-29 | Quizlet
Every isotope (at least, the ones that occur naturally) contributes to the average atomic mass, which appears in the element’s box on most periodic tables. But the average is what is called a weighted average. A weighted average mass is an average that takes into account how many times each mass occurs in a sample.

Source Image: quizlet.com
Download Image
SOLVED: Element X has two naturally occurring isotopes, 65X (isotopic mass 64.9797 amu, abundance 23.62%) and 67X (isotopic mass 67.1540 amu, abundance 76.38%). Calculate the atomic mass of element X. ???? amu *please show work clearly 🙂
Silicon has three naturally occurring isotopes (Si-28, Si-29 | Quizlet
1. rusting of a nail. 2. freezing of water. 3. decomposition of water into hydrogen and oxygen gases 4. compression of oxygen gas. 1,3. The element X has two naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu.
SOLVED: Element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below: What is the average atomic mass of the element? The Three Isotopes of Hydrogen | Differences & Properties – Video & Lesson Transcript | Study.com
Chemistry Periodic Table of Elements Chemistry-Chapter 2 Practice Problems An unknown element is found to have three naturally occurring isotopes with atomic masses of 35.9675 (0.337%), 37.9627 (0.063%), and 39.9624 (99.600%). Which of the following is the unknown element? Click the card to flip 👆 Ar Click the card to flip 👆 1 / 9 Flashcards Learn